Professor Leonie Quinn is a leading biomedical researcher at The Australian National University’s (ANU). Prof Quinn’s Brain Cancer Discovery Lab (Figure 1), located in the John Curtin School of Medical Research (JCSMR), is dedicated to advancing the fight against brain cancer. With their research focus on glioma – the most common and aggressive primary brain tumour – the Quinn lab seeks to uncover the genetic mechanisms driving tumour growth, which will enable them to develop new targeted treatments to improve outcomes for patients.
Lamentably, brain cancer remains one of the most difficult cancers to treat. More distressing is the lack of major breakthroughs in treatment for more than 40 years. The reasons are multi-fold but, in part, because brain cancer has not attracted sufficient funding. Indeed, past investment have improved patient survival for other cancer types (e.g. breast, prostate, colon), while the same advancements have not been made for brain cancer.
The Quinn Lab is steadfast in their mission to change this reality, with the knowledge that effective treatments will only come through sophisticated approaches to understand the brain. Indeed, the COVID-19 pandemic underscored how collaboration and dedication to new avenues of research can lead to transformative results in record time. With the same spirit, Prof. Quinn and her team are accelerating brain cancer research, and with the support of ABCF, are making a tangible difference. And, as for the success with combatting COVID-19, their innovative ideas from their multidisciplinary team are resulting in meaningful discoveries.
Gliomas remain difficult to treat, with survival rates stagnant for decades. We desperately need more researchers to think about the complexity, intricacy, and uniqueness of the human brain. Professor Quinn’s two decades working as a geneticist, solving the complex molecular and cellular mechanisms controlling development in living systems, provides her with a unique approach to brain cancer research. To improve treatment for complex diseases, like brain cancer, she is acutely aware that we must first understand the genetics of normal brain development. And, as every patient’s tumour is unique, we must understand the genetics of individual brain tumours, towards developing specialised treatments tailored to each patient. To achieve such personalised medicine for glioma patients, the Quinn lab use a unique combination of Drosophila genetics and human glioma (mini brain) models to unlock the mysteries of the developing brain and understand the genetics of brain cancer.

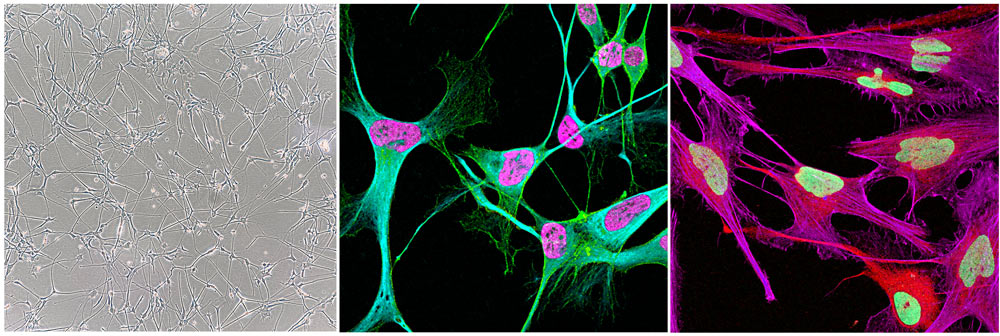
To unlock the mysteries of the brain, one avenue of Professor Quinn’s research employs the sophisticated genetics of Drosophila melanogaster (vinegar fly) (Figure 2). A model that has been used for >120 years to unlock the mysteries of animal development. The significance to human disease from discoveries made using in this tiny fly is highlighted by 6 Nobel Prizes for Drosophila in the field of Medicine and Physiology! Indeed, there are treatments now in clinic for many cancers informed by discoveries in Drosophila.
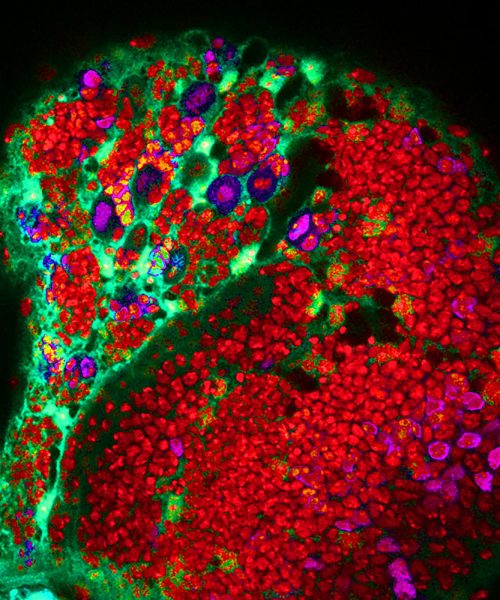
Thus, studies in the Drosophila brain (Figure 3) enable the team to determine how the unique mutations in each patient results in glioma. As this approach allows her team to study the behaviour of tumour cells in a controlled environment, they can determine the molecular changes that drive brain cancer. Thus, the team has identified the molecular and cellular basis for tumour formation driven by mutations identified in individual glioma patients. These discoveries provide the foundation for exploring potential therapeutic targets, addressing a critical gap in glioma treatment, which her team achieves using the “mini brains” systems (Figure 4).
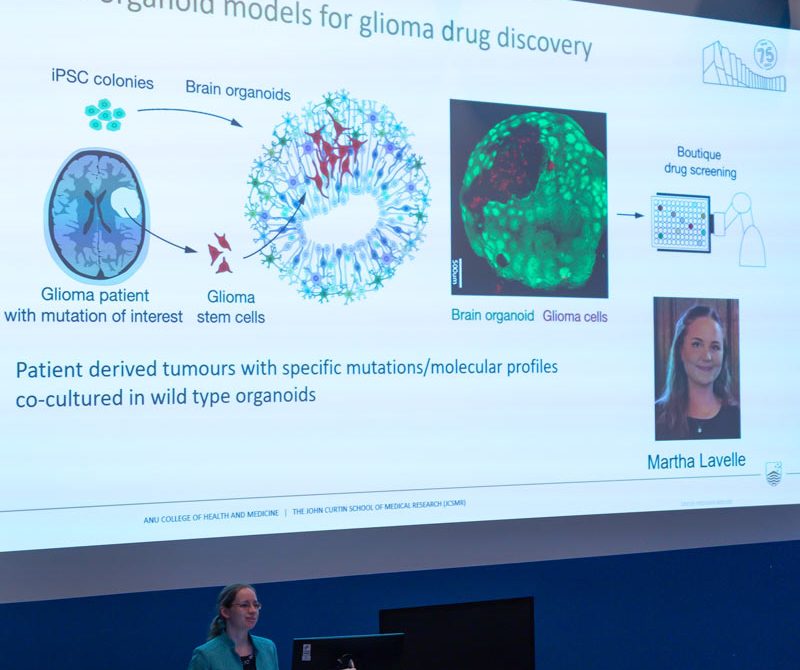
Personalised medicine requires screening of individual tumours to identify the best treatments for each patient. A groundbreaking aspect of Professor Quinn’s research is the use of 3D brain organoids, or ‘mini-brains,’ which replicate the structure and environment of the human brain, offering an innovative platform for testing unique drug therapies (Figure 4). To achieve their personalised medicine program, Prof. Quinn’s team capitalises on the support of state-of-the-art infrastructure, already established through hundreds of millions of dollars in grant funding from multiple agencies and ongoing support from the ANU and John Curtin School. This infrastructure is not only critical to implement the team’s cutting-edge research, but to also retain and attract the best researchers in the world to Canberra.
Thus, the team can couple their knowledge of brain tumour genetics, determined by next generation sequencing, with high-throughput screening to test thousands of drugs for their therapeutic potential against an individual glioma. First, the molecular analysis uses next generation sequencing for each patient’s glioma in association with ANU’s Biomolecular Resource Facility (BRF). With cellular aspects of each of glioma determined by advanced biomedical imaging through ANU’s Centre for advanced microscopy (CAM), a node of Microscopy Australia. The team has further established advanced bioprinting technology to generate mini-brains and, in collaboration with the ANU Centre for Therapeutic Discovery (ACTD), will screen thousands of new drugs for capacity to treat individual brain tumours (Figure 5). Thus, the team will capitalise on their biobanking and mini brain development to identify new, personalised treatments for each patient.
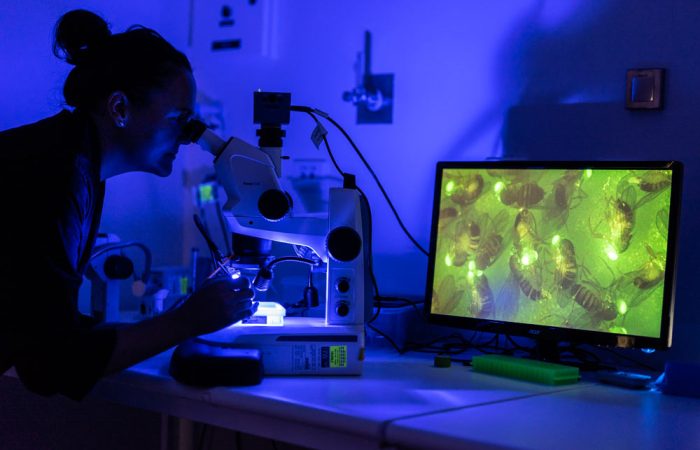
Professor Quinn’s work integrates genetic insights, advanced technology, and clinical collaboration to revolutionise brain cancer research. Her contributions bridge the gap between laboratory discoveries and patient care, ensuring that scientific advancements translate into better outcomes for patients.
Through initiatives like the CBCC and the ACT Brain Cancer Biobank, her research empowers the development of personalised therapies essential to improve survival rates and quality of life. Her commitment to advancing understanding and treatment of brain cancer inspires hope for patients and sets a high standard for future research.
Professor Leonie Quinn’s innovative work exemplifies the transformative potential of science in addressing the challenges of brain cancer, paving the way for breakthroughs that benefit patients and families alike.
The expedite her team’s discoveries to clinical trials and, ultimately, improve patient outcomes, Professor Quinn founded the Canberra Brain Cancer Collaborative (CBCC). This initiative links ANU’s research with clinical teams at The Canberra Hospital, fostering collaboration in oncology, neurosurgery, and other relevant fields. A major milestone of the CBCC was the establishment of the ACT Brain Cancer Biobank (ABCB) by Naomi Mitchell (Figure 6), Canberra’s first repository of brain cancer biospecimens and genomic data.
Funded by the ACT Health Research Innovation Fund, the biobank supports the development of personalised patient-derived tumour models (Figure 7). Importantly, the team’s expertise in next generation sequencing and genomic profiling of glioma samples, enables identification of precision brain cancer therapeutics tailored to each patient. As every patient’s tumour is genetically unique, the comprehensive collection established by the ABCB, is critical for understanding individual brain cancer genetics and facilitating discovery of personalised treatments.
Thus, the strong clinical-research network, comprising a multidisciplinary team of biomedical researchers (geneticists, molecular biologists, and bioengineers), and clinicians (neurosurgeons, oncologists, rad-oncologists, pathologists) is enabling the team to better understand the complexity of brain cancer. The CBCC will continue to work collaboratively to push boundaries, uncover new treatments, towards our commitment to offer hope by extend brain cancer patient survival.
Investments in our research enables Prof. Quinn to continue mentoring and inspiring the next generation of graduate students, driving the greatest minds to focus on solving the complexities of the human brain and the genetics of brain cancer (Figure 1).
In 2024, 3 talented honours students graduated from the Quinn Group with first-class honours; Sarah and Julia will continue their research as PhD candidates in brain cancer research in 2025, while Jithmi will commence medicine at ANU with the future ambition of a career in clinical aspects of brain cancer research. In previous years several honours students graduating from the Brain Cancer Discovery Group, also receiving first-class honours, were awarded Australian Government scholarships to embark on PhD studies. The most recent PhD graduate, Dr Damien Muckle, discovered novel pathways for regulating neural stem cell fate in the brain, which is of major significance to tumour progression. During his candidature Damien presented his work at leading international conferences (in New York and Paris), received his Doctor of Philosophy in October this year, and has a major first author manuscript in preparation for a prestigious international journal.
The Quinn Group are currently training 4 more extremely talented PhD students. Brooke Kinsela, Elizabeth Troy, and Tanya Javaid are due to graduate in 2025, 2026, 2027, respectively. More recently, the team were privileged to be joined by Martha Lavelle from the University of Bristol (UK), who brings her unique bioengineering expertise to help fast-track 3D-printing of brain organoids (mini brains) for drug screening. Recruiting such world-class expertise is enabling the critical knowledge exchange required to drive pioneering discoveries towards the holy grail of personalised brain cancer treatments.
In addition to graduate student training, Prof. Quinn is invested in mentorship of early career postdoctoral research fellows. Dr Olga Zaytseva (Figure 8), who is leading the mini brain project, completed honours and PhD studies with the Quinn lab (University of Melbourne), publishing numerous lead author manuscripts in leading international journals. Olga has also earned recognition through invitations to present her research at leading National and International Conferences, the award of prestigious prizes, fellowships and grants (e.g. the ANU/CHM Early Career Fellowship in 2021, JCSMR Gender Equity Award in 2022, and The ACT Cancer Council Grant in 2023), while balancing care for her son Arthur (Figure 9). Nan-Hee Kim also recently returned from maternity leave, after her son Roan was born in 2023, and is now leading the Next Generation Sequencing of the patient samples collected by the ACT Brain Cancer Biobank. The genetic and genomic data generated by Nan-hee provides the basis for understanding molecular mechanisms of tumour initiation and progression, which is essential for breakthroughs in drug discovery.
Thus, the brain cancer research program is enabling training of the brightest minds to continue innovative brain cancer research and dissemination of knowledge to the next generation.